13 Best Clinical Trial Management Software for 2026

Sorry, there were no results found for “”
Sorry, there were no results found for “”
Sorry, there were no results found for “”

Clinical trials are complex.
They involve several aspects that need attention—from recruiting diverse patient populations to managing studies, data analysis, reporting, monitoring logistics, and more.
Even if you miss out on a single protocol amendment, the result will be non-compliance, hefty penalties, and legal issues.
This is where Clinical Trial Management Systems (CTMS) come in to bring all the scattered processes under one roof. A CTMS platform ensures all critical processes are in one place, streamlining clinical trials and research operations and helping you make informed decisions faster.
This listicle includes the best clinical trial management software designed to improve your clinical trials. Explore them all and choose the ideal one that serves your purpose.
Here’s a comparison table for the Clinical Trial Management Systems (CTMS):
| Tool Name | Best For | Key Features | Pricing |
| ClickUp | Managing trial documentation, task tracking, and research workflows | Task tracking, document collaboration, automation, dashboards, HIPAA compliance, AI-powered insights | Free forever; Customizations available for enterprises |
| Zelta | Clinical data management | Real-time trial data insights, predictive resupply analytics, KPI-based dashboards | Custom pricing |
| Florence eBinders | Remote trial monitoring and management | eISF, e-signatures, document storage, remote monitoring, built-in redaction controls | Custom pricing |
| Clinion | Centralizing and automating clinical operations | AI/ML modules, protocol automation, IP inventory management, real-time visibility | Custom pricing |
| Veeva Vault | Centralizing clinical data and content | Study registration sharing, eCOA, centralized study communications | Custom pricing |
| Medidata | Study management and data visualization | Automated data transfers, visual analytics, eTMF and Rave EDC integrations | Custom pricing |
| Oracle Siebel | Monitoring and managing clinical trial operations | Workflow automation, personalized portals, auto-tracking subject visit completion | Custom pricing |
| MasterControl CTMS | Clinical documentation and quality management | TMF and GCP document management, site qualification and monitoring, integration of clinical operations and quality management | Custom pricing |
| TrialKit | Managing remote trials and decentralized studies | EDC, RTSM, AI-powered analytics, native mobile app | Starting from $1,350 onwards |
| RealTime CTMS | Streamlining patient enrollment and clinical trials | Patient tracking, automated scheduling, HIPAA compliance, advanced reporting | Custom pricing |
| Edge CTMS | Real-time clinical research management | Workflow builder, finance module, patient recruitment tracking | Custom pricing |
| Castor EDC | Capturing clinical trial data | eCRFs, real-time study insights, role-based access, survey features | Custom pricing |
| Clinical Conductor CTMS | Optimizing recruitment and financial workflows | Recruitment tracking, financial management, centralized study information | Custom pricing |
Clinical Trial Management involves planning, executing, and monitoring clinical research to ensure it is ethically conducted in compliance with industrial regulatory standards.
Managing clinical trials requires coordinating several aspects, such as patient recruitment, data collection, data-driven decision-making, document management, budget management, maintaining compliance, and reporting across sites and stakeholders. Here, the goal is to perform the trial efficiently while maintaining data integrity and the safety of participants.
🧠 Did you know? According to a research study, conducting successful clinical trials requires various factors, including project planning, seamless collaboration, minimal work for investigators and participants, efficient systems, and proper recruitment of trial participants.
Whether you’re looking for clinical trial management software or a platform to help you create utilization management processes, make sure the software has these features:
💡Pro Tip: Consider the platform’s user-friendliness and scalability. Make sure that the software can accommodate your growing trial volumes and complexities. This will help you choose software that can be sustained as your research evolves.
Let’s see the best CTMS software, their key features, pricing, and limitations.
Be it on-site, remote, or hybrid, clinical trials mean a lot of work—onsite visits, site monitoring, overwhelming documentation, complex trial processes, and strict regulatory requirements, resulting in disconnected workflows.
This is where ClickUp, the everything app for work, combines project, knowledge, and chat in one place—all powered by AI that helps make your clinical trials faster and more efficient.
ClickUp for Healthcare can improve patient experiences with automatic appointment scheduling and contract and SLA processes, fast-tracking record requests, and collaborating on patient data and research. It is a SOC 2 and HIPAA-compliant platform with advanced user permissions for data protection.

To start with, ClickUp Tasks streamlines clinical trials. It enables you to organize complex trial workflows into manageable tasks (with checklists), monitor trial supplies, assign responsibilities, and track clinical trial progress in real time. You can also Assign Comments to specific team members to ensure accountability during the trial phases.
ClickUp Docs make clinical documentation hassle-free. It serves as a central repository for storing and managing regulatory documents, research notes, and study reports. Trial research teams can easily collaborate within these documents, maintaining version control.

When it comes to processes, offload the burden of routine trial processes such as routing approvals, study reminders, changing task and data collection status, and follow-up task alerts with ClickUp Automations. You can also integrate ClickUp with other CTMS platforms to create trigger-based automation rules and make files searchable within a single, integrated workspace.
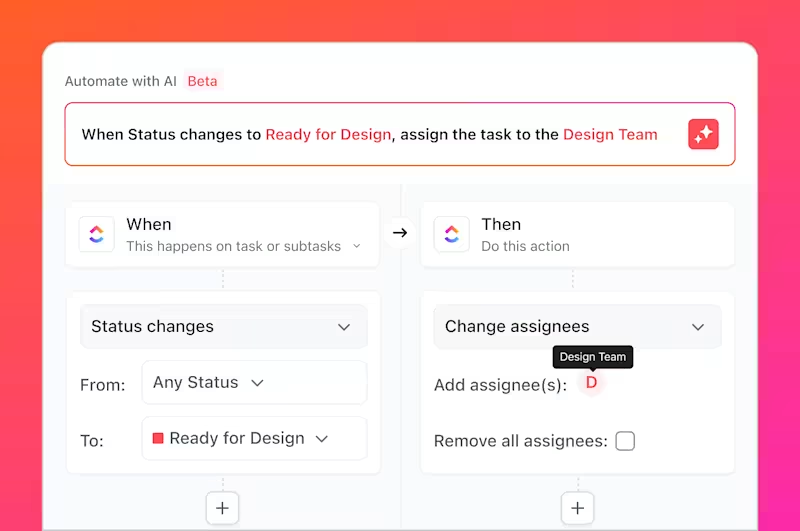
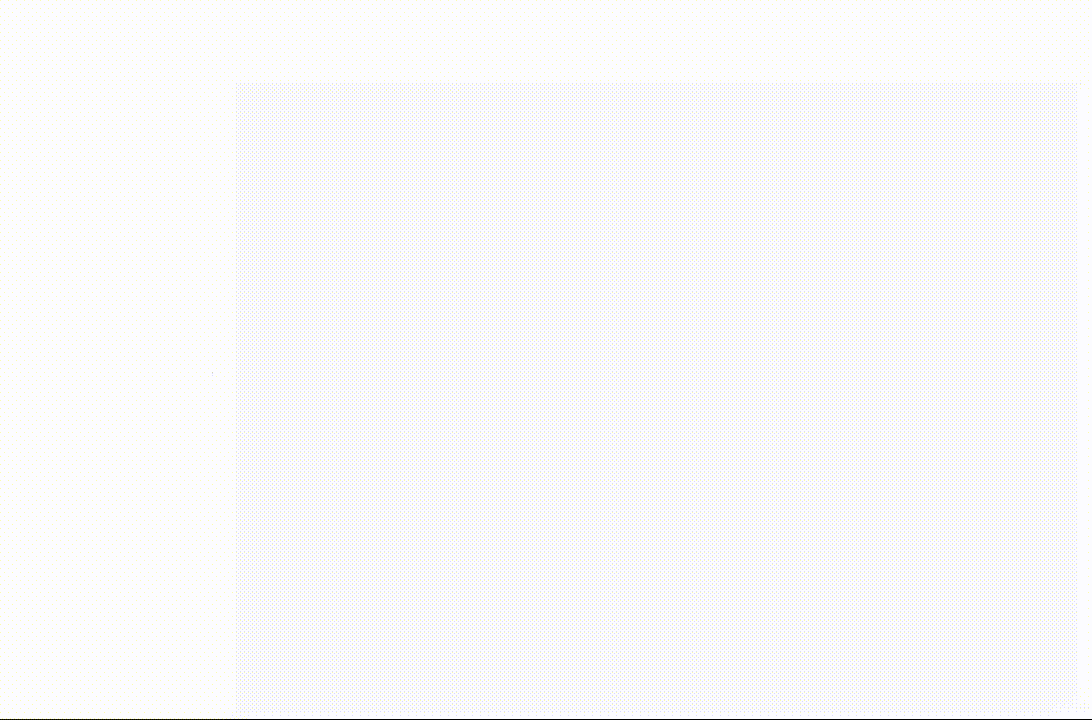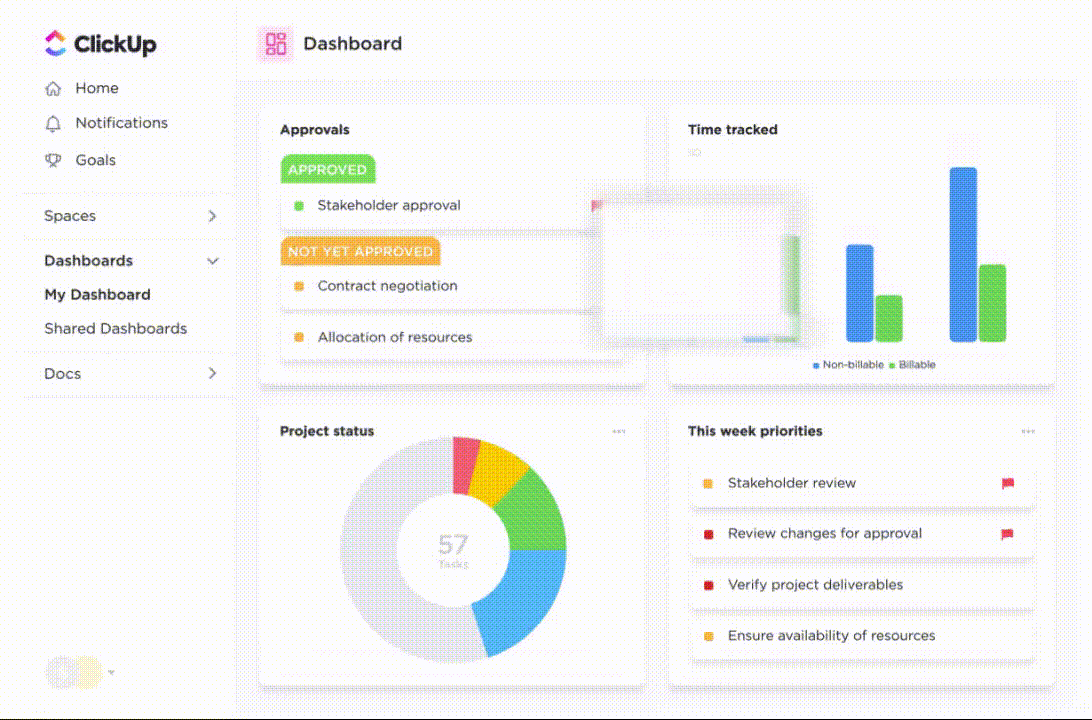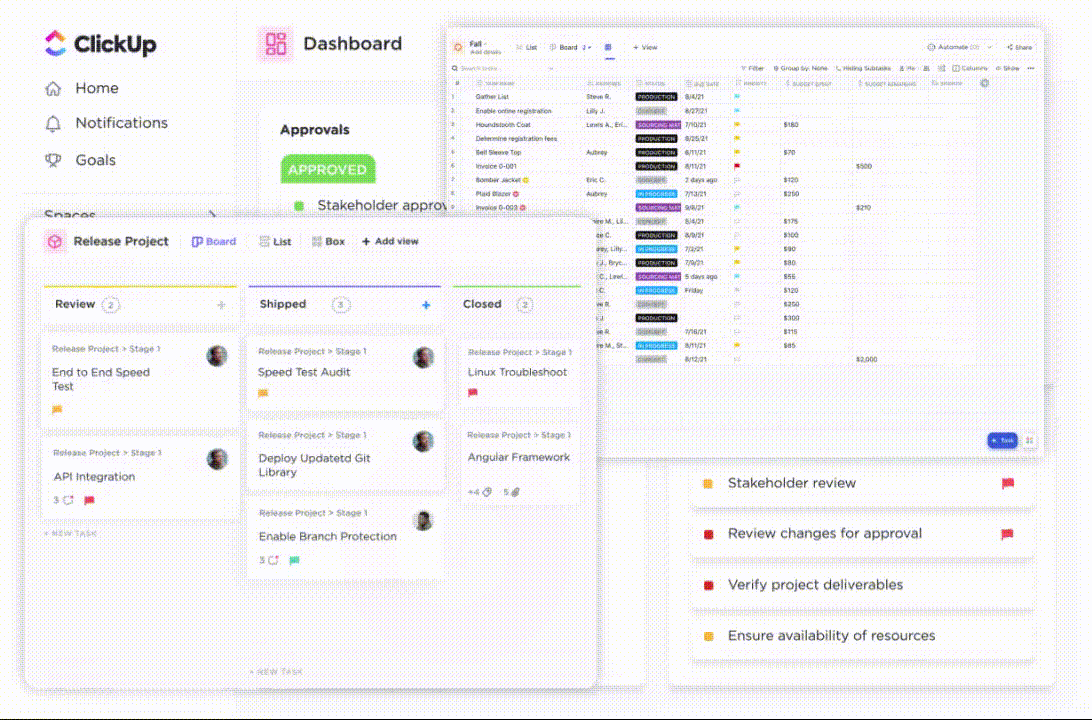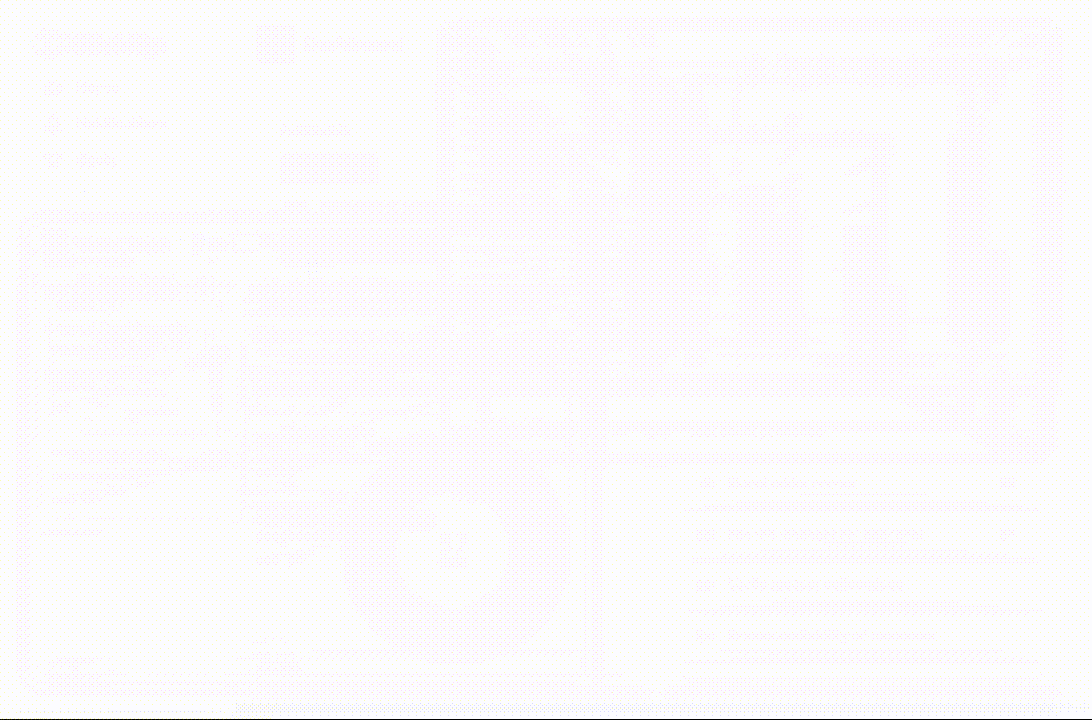
ClickUp also helps you monitor trial timelines and compliance checklists and helps you gain visibility into team productivity metrics. With customized ClickUp Dashboards, you can quickly access essential trial metrics and details without digging through heaps of data.
Besides these features, ClickUp makes your work easier with a huge template library. For instance, the ClickUp Patient Management Template helps you:
Similarly, ClickUp has several process documentation templates to help you standardize processes and ensure consistency and transparency.

ClickUp is a powerful tool where the information about every project in our organization can be centralized for multi-disciplinary and regional teams to keep everyone on the same page as the business is moving towards progress.
💡Pro Tip: Learn how to set up ClickUp Dashboard for clinical trial project management.👇

Zelta, by Merative Clinical Development (formerly, IBM Clinical Development), is a clinical data management platform that makes clinical trials more efficient. It has a customizable approach that allows you to select functionalities for specific studies.
With Zelta, you can gain insights into real-time trial data, including participant engagement, regulatory compliance, and more, in one place. It also comes with trial supply management and randomization features that help with even participant distribution and supply logistics optimization.
Florence eBinders supports clinical research with eISF (electronic Investigator Site Files) and participant binders. It offers features like e-signature and document storage to accelerate trial management.
The software’s advantage lies in its remote monitoring capabilities. These features allow you to monitor trial activities in real time without the on-site-visit hassles, review priority documents, and track compliance on the go. Also, you can directly access redacted participant binders without uploading duplicate docs to sponsor portals.
Easy to use app with awesome features that simplify and make work easy. However, it still has some complications when it comes to using it, you still need to be an experienced tech worker.
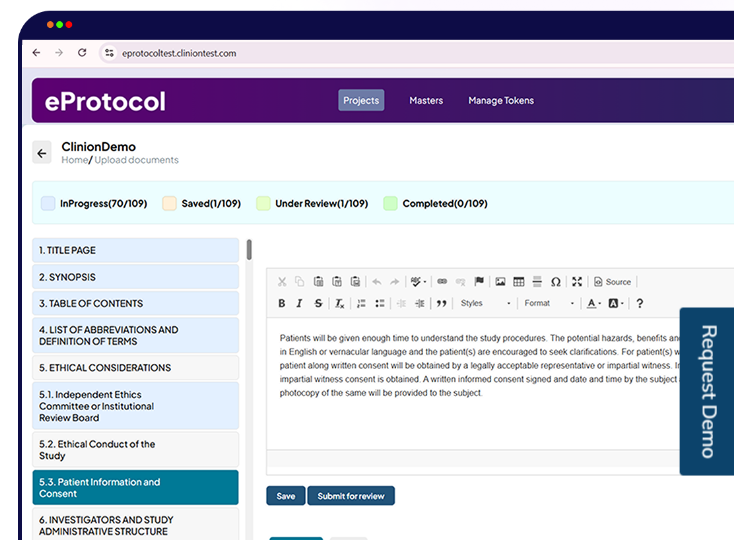
Clinion’s eClinical platform integrates trial management, EDC, RTSM, eConsent, eProtocol, CSR automation, and eTMF (Electronic Trial Master File) to streamline clinical trials. One of its standout features is the real-time visibility that allows you to monitor every step of the trial—from subject recruitment to data capture and course corrections.
It also has integrated AI/ML modules, such as AI medical coding, to speed up trial timelines and enhance data quality and compliance.
📖 Read More: Best AI Tools in Healthcare
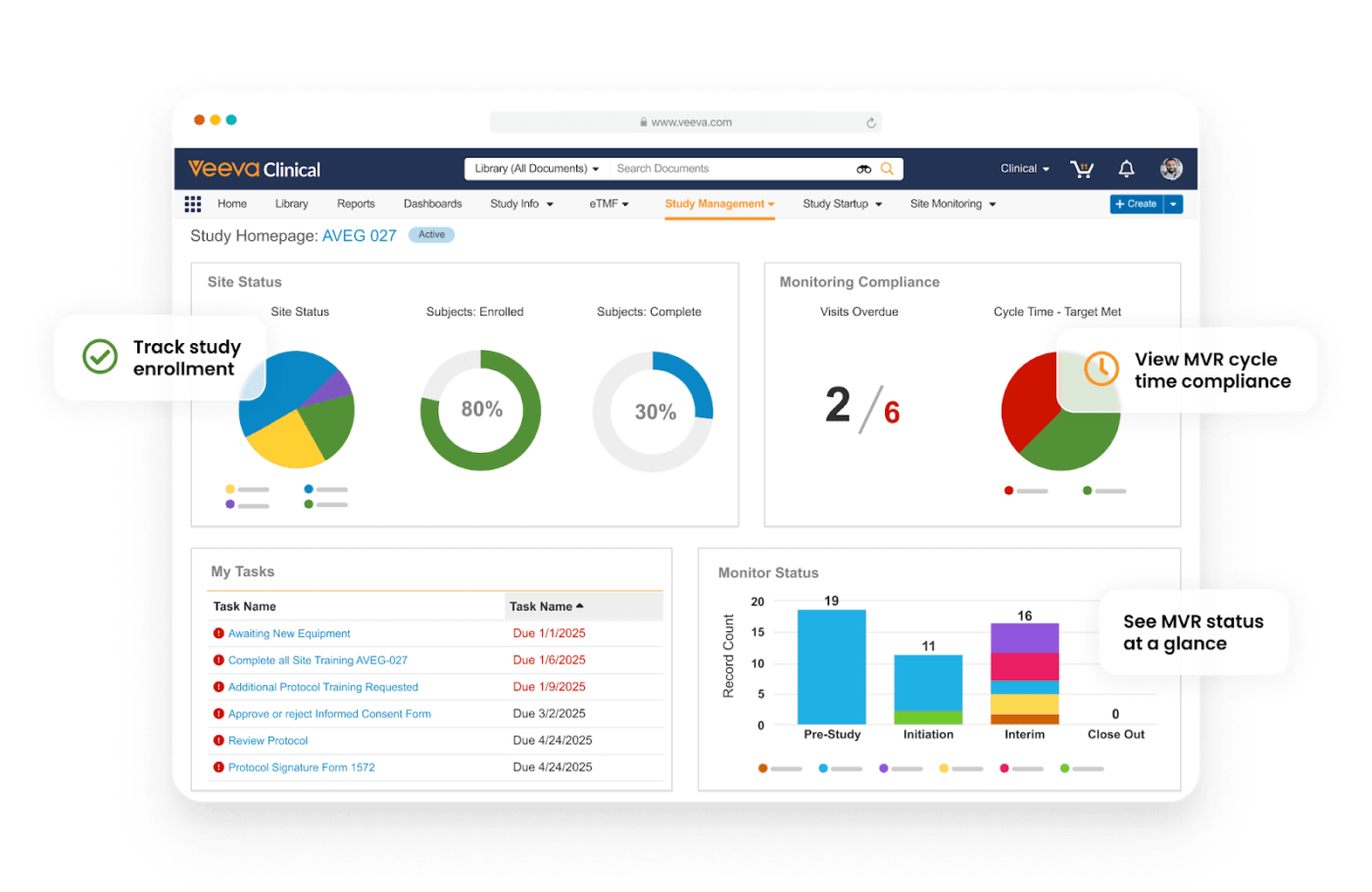
Veeva Vault is a trial management software with the features to meet your healthcare project management needs. It supports real-time collaboration with editing, commenting, and integrated chat. It allows you to track approvals for content and documents, ensuring regulatory compliance and standardized workflows.
The platform’s main highlight is that it serves as a single source of truth for clinical data and documentation. It directly connects with other Veeva apps, facilitating real-time insights, operational efficiency, and prompt decision-making.
The overall experience was satisfactory and I was able to use collaboration features very well and keeping track of the task was easy. However, the thing that is not good is that its performance is slow with large datasets and there are latency issues.
📖 Read More: Best Healthcare Project Management Software
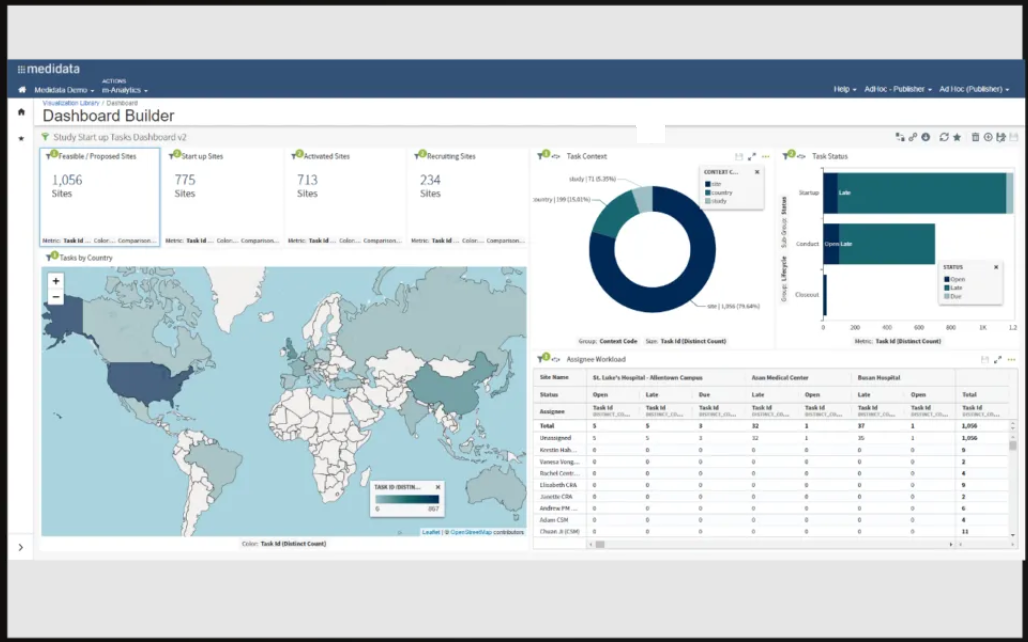
Medidata’s CTMS is a straightforward tool with automated workflows that helps you streamline manual clinical processes. The software’s seamless integration with eTMF and Medidata Rave EDC eliminates manual data entry, allowing you to manage trials, research studies, and sites. Additionally, it offers powerful visual analytics to monitor studies and trial processes.
Overall, Medidata is fairly easy to use with comprehensive training videos. The interface is not fun or intuitive to navigate and completed pages do not flow into the next page once saved, making data entry more time-consuming. Also, I don’t like that when you finish a data entry page of the eCRF, after I save, it doesn’t automatically flow to the next page where data is needed, especially in the same visit.
📮ClickUp Insight: We recently discovered that about 33% of knowledge workers message 1 to 3 people daily to get the context they need. But what if you had all the information documented and readily available?
With ClickUp Brain’s AI Knowledge Manager by your side, context switching becomes a thing of the past. Simply ask the question right from your workspace, and ClickUp Brain will pull up the information from your workspace and/or connected third-party apps!
Oracle Siebel’s CTMS helps you enhance clinical trial operations, maintain trial quality, and better manage investigator relationships.
The platform has robust tools, such as trip reports, document management, site payment tracking, and calendar management, for running clinical trials more efficiently. It also offers advanced trial status and management reports to improve decision-making.
📖 Read More: Best Clinic Management Software
MasterControl combines CTMS and CQMS (Clinical trial and quality management systems) so you can manage everything — from collecting patient data to documentation, quality, and risk— improving data accuracy and efficiency in clinical trials.
The software streamlines the management of your eTMF, docs, activities, quality events, audits, training, and projects throughout a clinical trial. It simplifies information exchange using several data importing and exporting methods. Plus, it allows you to link, report, and extensively research each site’s information, including study qualifications and participation, audit histories, and monitoring visits.
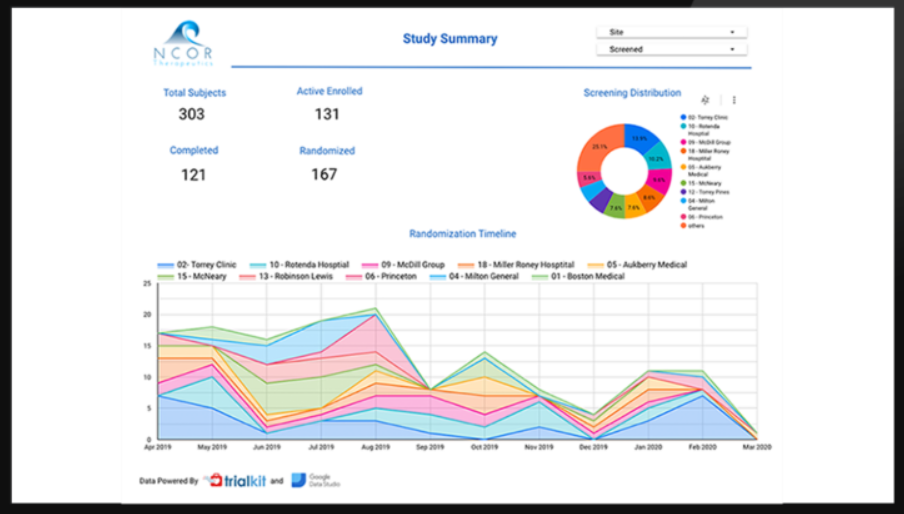
TrialKit is an end-to-end clinical trial management system with resource management that simplifies running trial executions. It allows real-time data entry and direct data transfers into the EDC app, accelerating data collection.
The platform automates electronic consent processes and patient-reported outcomes, helping you conduct remote and hybrid clinical trials. Also, TrialKit offers AI-powered analytics to help you make informed decisions. The platform has a native mobile app that allows researchers to manage trials anywhere.
TrialKit meets our research study needs. The user interface is great, enabling users to work around the platform easily. Their comprehensive knowledge base comes with AI that can answer your questions directly. Customer support is exceptional! We never had a question that went unanswered, and they also extend 1:1 consultation calls to help you with almost anything. However, it lacks features, such as an easier way to design the homepage, more flexibility with the dashboard & homepage reports, and more advanced conditional actions.

RealTime CTMS helps you streamline clinical trials, patient enrolment, and study management. It comes with scheduling tools that automate alerts on study target dates and upcoming events. It simplifies patient tracking and has advanced reporting capabilities to help you monitor site performance, recruitment, financial metrics, and staff productivity.
RealTime CTMS is also HIPAA-compliant software that helps ensure your clinical trials comply with current regulations and ethical standards with features like audit trails and data integrity checks.
Real Time-CTMS offers a set of features that help clinical trial management. I also love the training program that covers every user’s needs, and the customer service deserves a 5-star rating. However, I wish there could be a way to import study visits. It would be such a time saver and minimize the risk of errors during data entry.

Like most CTMS, Edge is built as clinical trial and patient management software. The software specializes in helping researchers track and manage their studies from start to end. Plus, it provides complete visibility and control of patient recruitment.
Edge CTMS has other beneficial features, such as a financial module and custom workflows. Hence, it can replace your spreadsheets to provide a more transparent view of financial reports, workflows, patient attributes, invoices, and more.
Great system – easy to use, easy to train others to use, currently could not do my job without it. I am evangelical about EDGE – it’s a great design! However, the system logs you out if no keystrokes/mouse movements after a very short period.
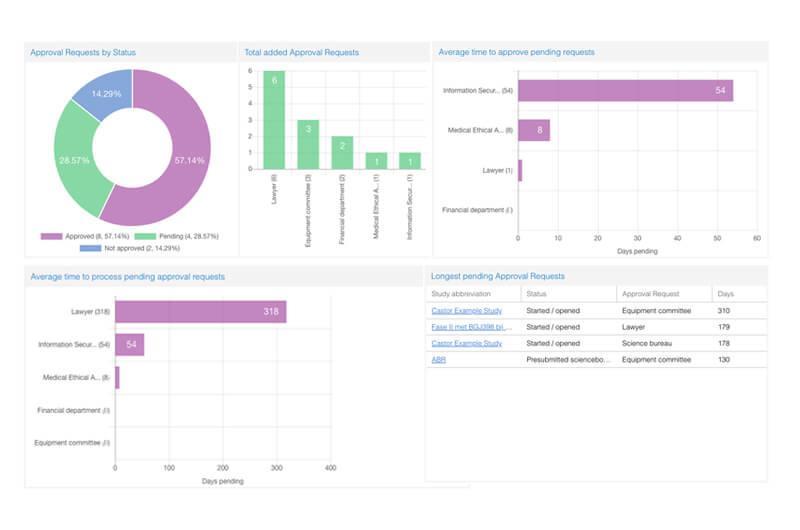
Castor EDC is a cloud-based CTMS and data entry software. Its user-friendly database is great for tracking patient records. You can enter data, generate automatic queries, and use the various filter options to search data quickly. The platform also shows the overall patient journey, their progress, and outstanding work—all in one place.
Castor’s highlight is its elaborate survey features. You can build case forms for trials and clinical research, create various surveys using templates, send web surveys, organize surveys into packages for easy distribution, and also display a form navigator on the surveys for participants to easily navigate between sections.
Castor EDC is a user-friendly, and easy-to-operate tool in clinical trials that I have used so far. It provides flexibility in using it. It allows changes in features that can be added to accommodate new needs. However, it is centrally controlled. It is challenging to provide narrations to some slots which are restricted. On the other hand, it operates slowly on some days.
Clinical Conductor CTMS, by Advarra, offers robust clinical study tracking, recruitment, compliance, and financial capabilities. It has a huge database with filtering options and custom dashboards, making it apt for handling thousands of patients.
The platform helps track patient recruitment, create detailed reports, and organize meetings and appointments. The standout feature of the tool is its patient scheduling. You simply enter each study, set intervals, enroll, and schedule.
Clinical Conductor CTMS is wonderful for trial management, finance, recruitment, and scheduling. Once you learn the interface, there are lots of ways to view information. One downside is it does not offer electronic source or document management. If it did, it would truly be a one-stop shop for clinical trial sites.
Without technology, modernizing clinical trials is like a distant dream. Whether you want to organize documentation, enhance monitoring, or speed up processes, you need the ideal CTMS.
Our listicle has the top CTMS with varied features. All you must do is assess your needs, priorities, and budget to choose a platform that fits your bill. For instance, if your priority is research management, consider Edge CTMS, and if you’re dealing with remote trials, shortlist Florence eBinders.
But if you want a comprehensive solution with all project management aspects to streamline and accelerate your clinical trial processes, try ClickUp.
Sign up for free today to see ClickUp in action!
© 2026 ClickUp