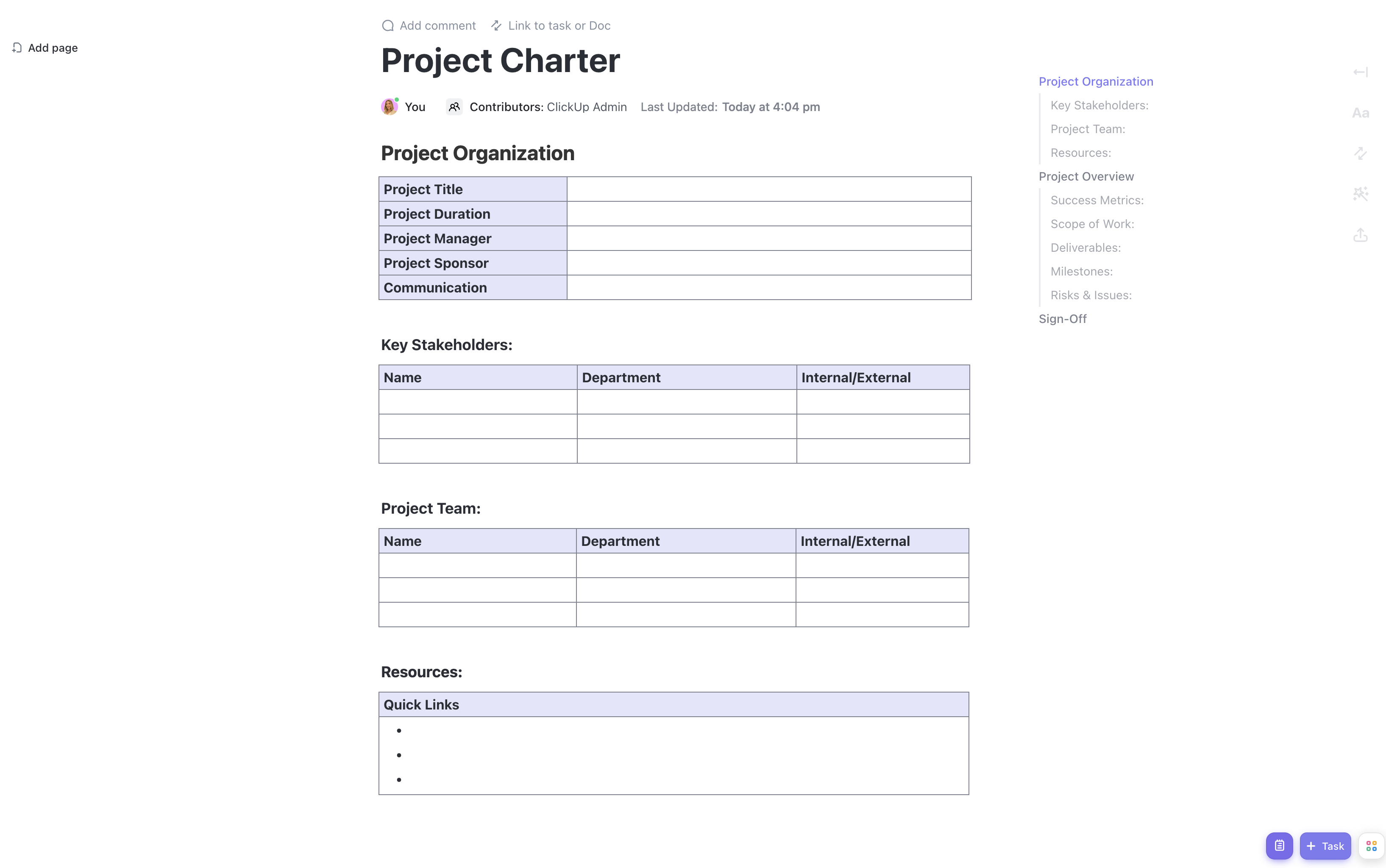
When it comes to conducting clinical trials, having a solid plan is essential for success. From defining objectives to managing timelines, there are countless moving parts to consider. That's where ClickUp's Clinical Trial Project Charter Template steps in to save the day!
With this template, you can:
- Clearly outline project goals, deliverables, and timelines
- Define roles and responsibilities for your research team
- Track progress and milestones to ensure everything stays on track
- Collaborate and communicate seamlessly with your team
Whether you're conducting a groundbreaking medical study or managing multiple trials, ClickUp's Clinical Trial Project Charter Template has everything you need to streamline your process and achieve your research goals. Get started today and experience the power of efficient clinical trial management!
Benefits of Clinical Trial Project Charter Template
When it comes to clinical trials, having a solid project charter is essential. The Clinical Trial Project Charter Template offers a range of benefits, including:
- Streamlining the planning process by providing a clear structure and framework
- Ensuring all stakeholders are aligned on project goals, timelines, and deliverables
- Facilitating effective communication and collaboration among team members
- Enhancing project transparency and accountability
- Providing a comprehensive overview of the project scope, objectives, and risks
- Saving time and effort by eliminating the need to create a project charter from scratch
Main Elements of Clinical Trial Project Charter Template
ClickUp's Clinical Trial Project Charter template is designed to streamline the process of creating and managing clinical trial projects. Here are the main elements of this Doc template:
- Custom Statuses: Track the progress of your clinical trial project with custom statuses tailored to your specific workflow, such as Planning, In Progress, Completed, and Review.
- Custom Fields: Capture important information about your clinical trial project using custom fields like Study Name, Principal Investigator, Start Date, End Date, and more. These fields allow you to easily organize and filter your project data.
- Different Views: Access your clinical trial project charter in various views, including Document view for a comprehensive overview, Table view for a structured and organized layout, and Calendar view for a visual representation of project milestones and deadlines.
With ClickUp's Clinical Trial Project Charter template, you can efficiently manage and document all aspects of your clinical trial projects, ensuring smooth execution and compliance.
How to Use Project Charter for Clinical Trials
When embarking on a clinical trial, it's crucial to have a well-structured project charter in place to guide your team and ensure a successful study. Follow these six steps to effectively use the Clinical Trial Project Charter Template in ClickUp:
1. Define the objective and scope
Clearly articulate the objective of your clinical trial project. What are you hoping to achieve with this study? Additionally, determine the scope of the project by outlining the specific parameters, such as the target population, interventions, and outcome measures.
Use the Goals feature in ClickUp to define and track the objective and scope of your clinical trial project.
2. Identify stakeholders and roles
Identify all the key stakeholders involved in the clinical trial, including researchers, sponsors, regulatory bodies, and participants. Assign roles and responsibilities to each stakeholder to ensure everyone understands their contributions and can work together seamlessly.
Create custom fields in ClickUp to document and track the roles and responsibilities of each stakeholder involved in the clinical trial.
3. Develop a timeline and milestones
Establish a timeline for your clinical trial, including key milestones and deadlines. This will help you stay on track and ensure that all necessary activities are completed within the desired timeframe. Consider factors such as recruitment, data collection, analysis, and reporting.
Utilize the Gantt chart feature in ClickUp to create a visual representation of your clinical trial timeline and set important milestones.
4. Define data collection and analysis procedures
Outline the specific procedures for data collection and analysis in your clinical trial. This includes the selection of appropriate data collection methods, such as surveys, interviews, or medical tests, as well as the statistical analyses that will be performed on the data.
Use the Docs feature in ClickUp to create detailed documentation of your data collection and analysis procedures for easy reference and collaboration.
5. Establish risk management strategies
Identify potential risks and challenges that may arise during the course of your clinical trial. Develop strategies to mitigate these risks and ensure the safety and well-being of participants. This may involve implementing safety protocols, obtaining necessary approvals, and monitoring adverse events.
Set up Automations in ClickUp to receive notifications and alerts for any potential risks or issues that may arise during the clinical trial.
6. Monitor and review progress
Regularly monitor the progress of your clinical trial and review the data collected to ensure that the study is proceeding according to plan. Make any necessary adjustments or modifications to the project charter as new information becomes available or circumstances change.
Use the Dashboards feature in ClickUp to track and visualize the progress of your clinical trial, including participant recruitment, data collection, and analysis.
By following these six steps and utilizing ClickUp's features, you can effectively use the Clinical Trial Project Charter Template to guide your clinical trial and ensure its success.
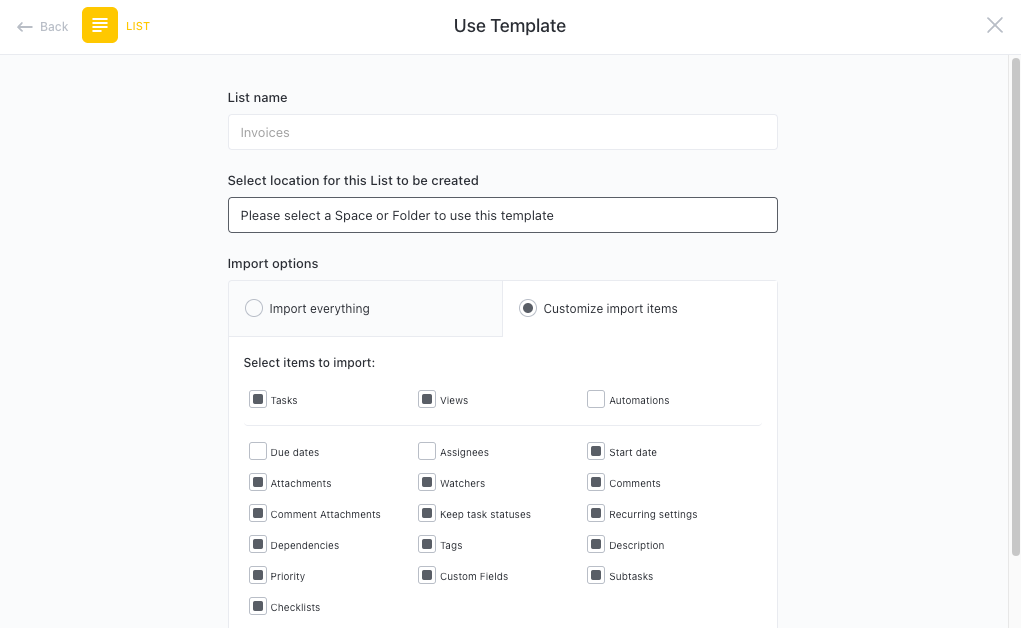
Get Started with ClickUp's Clinical Trial Project Charter Template
Medical researchers and clinical trial teams can use this Clinical Trial Project Charter Template to keep track of important details and milestones throughout the trial process.
First, hit “Get Free Solution“ to sign up for ClickUp and add the template to your Workspace. Make sure you designate which Space or location in your Workspace you'd like this template applied.
Next, invite relevant members or guests to your Workspace to start collaborating.
Now you can take advantage of the full potential of this template to manage your clinical trial project:
- Use the Gantt chart view to visualize the project timeline and dependencies
- The Board view will help you track the progress of each participant in the trial
- Utilize recurring tasks to set up regular check-ins and assessments for participants
- Create automation rules to streamline repetitive tasks and notifications
- Track important trial milestones using the Milestones view
- Use the Email integration to communicate with participants and stakeholders
- Leverage AI-powered analytics to gather insights from trial data
- Utilize ClickUp integrations to seamlessly connect with other tools and platforms
- Monitor team workload and resource allocation using the Workload view
- Collaborate on documents and protocols using the Docs feature
- Keep track of trial schedules and appointments using the Calendar view