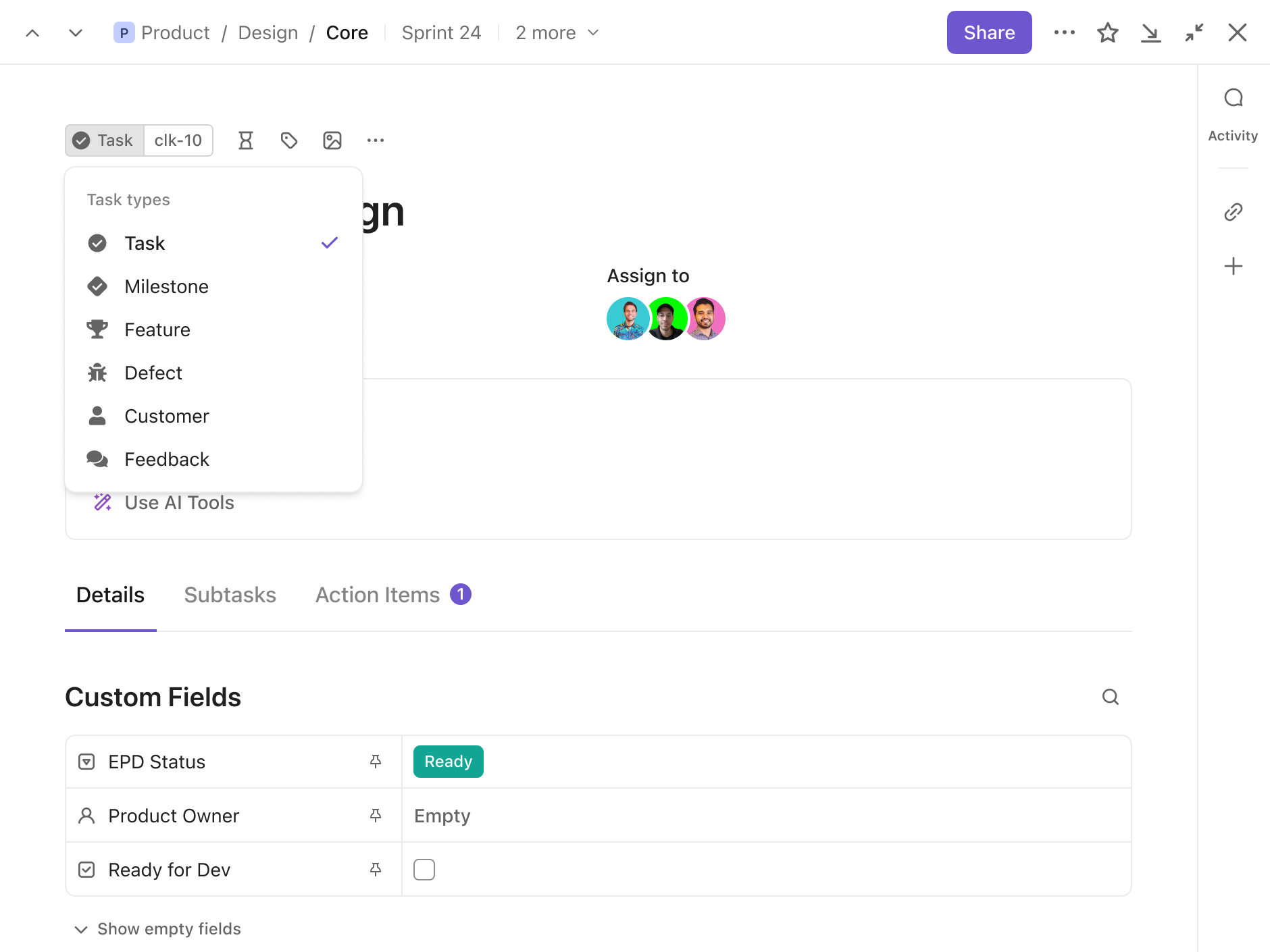
Custom Task Types
Manage any type of work
Customize your Workspace and manage any type of work in ClickUp. Use your own naming conventions and define the task types that make the most sense for your team.
Gantt Charts
Boost productivity and streamline workflows with the best task management software for Pharmaceutical Companies using ClickUp. Easily assign tasks, track progress, and collaborate seamlessly with your team to ensure projects are completed on time and within budget. Take your project management to the next level with ClickUp's powerful features designed to help pharmaceutical companies stay organized and efficient. Sign up now to experience the difference ClickUp can make for your team!
Free forever. No credit card.
Custom Task Types
Customize your Workspace and manage any type of work in ClickUp. Use your own naming conventions and define the task types that make the most sense for your team.

Task Types
Identify tasks for bugs, sprints, people, and more with Item Types. Group tasks by category to build a database for different Task Types.

Key features of task management software essential for pharmaceutical companies include robust scheduling capabilities for managing clinical trials and drug development timelines, secure data storage to adhere to regulatory requirements, collaboration tools for cross-functional team communication, and integration with project management tools for seamless workflow coordination.
Task management software can help pharmaceutical companies improve project efficiency and productivity by providing centralized task tracking, scheduling, and collaboration tools. This enables better resource allocation, timeline management, and real-time progress monitoring, leading to streamlined workflows and enhanced project outcomes.
Yes, task management software for pharmaceutical companies may include specific regulatory compliance features such as audit trails, electronic signatures, data encryption, and access controls to ensure adherence to industry regulations like HIPAA and FDA guidelines.