Task Management for Biotech Innovators
Task Management Software Tailored for Biotech Teams
Centralize experiments, monitor project milestones, collaborate effortlessly, and maintain full transparency throughout your biotech development cycles.
Trusted by the world’s leading businesses
Challenges in Biotech Project Management
Navigating Complexity: Why Biotech Teams Need Specialized Task Management
Without a dedicated system, biotech projects face fragmented data, missed regulatory steps, and inefficient collaboration — complicating even routine workflows.
- Extended development timelines obscure progress — making it difficult to track experiments, validations, and approvals.
- Data silos hinder innovation — critical datasets and protocols scattered across platforms reduce reproducibility.
- Compliance risks grow — tracking regulatory submissions and quality controls manually leads to errors.
- Cross-functional teamwork stalls — unclear responsibilities and version conflicts delay deliverables.
- Tight deadlines pressure submissions — grant applications, patent filings, and clinical milestones get overlooked.
- Progress visibility diminishes — long projects lack clear status updates, stalling decision-making.
- Communication breakdowns occur — emails and chats fail to keep multidisciplinary teams aligned.
- Resource scheduling conflicts arise — lab equipment and personnel availability overlap, disrupting workflows.
Comparing Workflows
Why Legacy Systems Can't Keep Up with Biotech Demands
Discover how ClickUp transforms task management beyond traditional tools.
Conventional Methods
- Tasks and data scattered across spreadsheets, emails, and disconnected apps
- Manual tracking of experiments and protocols prone to errors
- Compliance documentation handled in siloed files
- Collaboration slowed by unclear task ownership
- Deadlines for patents and regulatory filings often missed
- Disorganized document storage across drives and messaging platforms
ClickUp for Biotech
- Unified task management with clear statuses and priorities
- Centralized protocol templates and data attachments
- Automated compliance checklists and audit trails
- Transparent task assignments with real-time collaboration
- Automated reminders synced with calendars for critical deadlines
- Searchable, centralized documentation linked to every task
Use cases
Unlocking Efficiency: How Task Management Drives Biotech Success
See how streamlined task tracking reduces errors, enhances compliance, and accelerates innovation.
Consolidating Experimental Data and Protocols
ClickUp centralizes datasets, protocols, and notes, ensuring your team accesses up-to-date, searchable information linked directly to tasks and projects.
Maintaining Transparent Audit Trails for Regulatory Compliance
Track every step from sample collection to reporting with detailed timelines, comments, and file histories—supporting FDA and EMA audits seamlessly.
Managing Iterative Feedback on Research and Development
Capture stakeholder input with clear comments, version control, and task updates to keep development aligned and responsive.
Preventing Protocol Deviations in Critical Experiments
Utilize templates, checklists, and dependencies to enforce consistent execution and documentation of lab procedures.
Tracking Regulatory Submissions and Compliance Deadlines
Organize IRB approvals, patent filings, and quality assurance steps with custom workflows and automated reminders to avoid costly delays.
Streamlining Complex Bioinformatics Pipelines
Map and monitor data analysis workflows with statuses and dependencies, ensuring timely and error-free processing.
Coordinating Multi-site Clinical Trial Milestones
Manage timelines, documentation, and communications across global sites with centralized task boards and progress tracking.
Eliminating Redundancies in Literature and Patent Reviews
Track reviewed papers and patent documents with tags and notes to prevent duplicate efforts and surface critical insights.
Transforming Meetings Into Clear, Actionable Plans
Convert team discussions into assigned tasks with clear deadlines and checklists to ensure follow-through and accountability.
Elevate Every Phase of Your Biotech Projects
From discovery to clinical trials, keep your workflows precise and compliant.


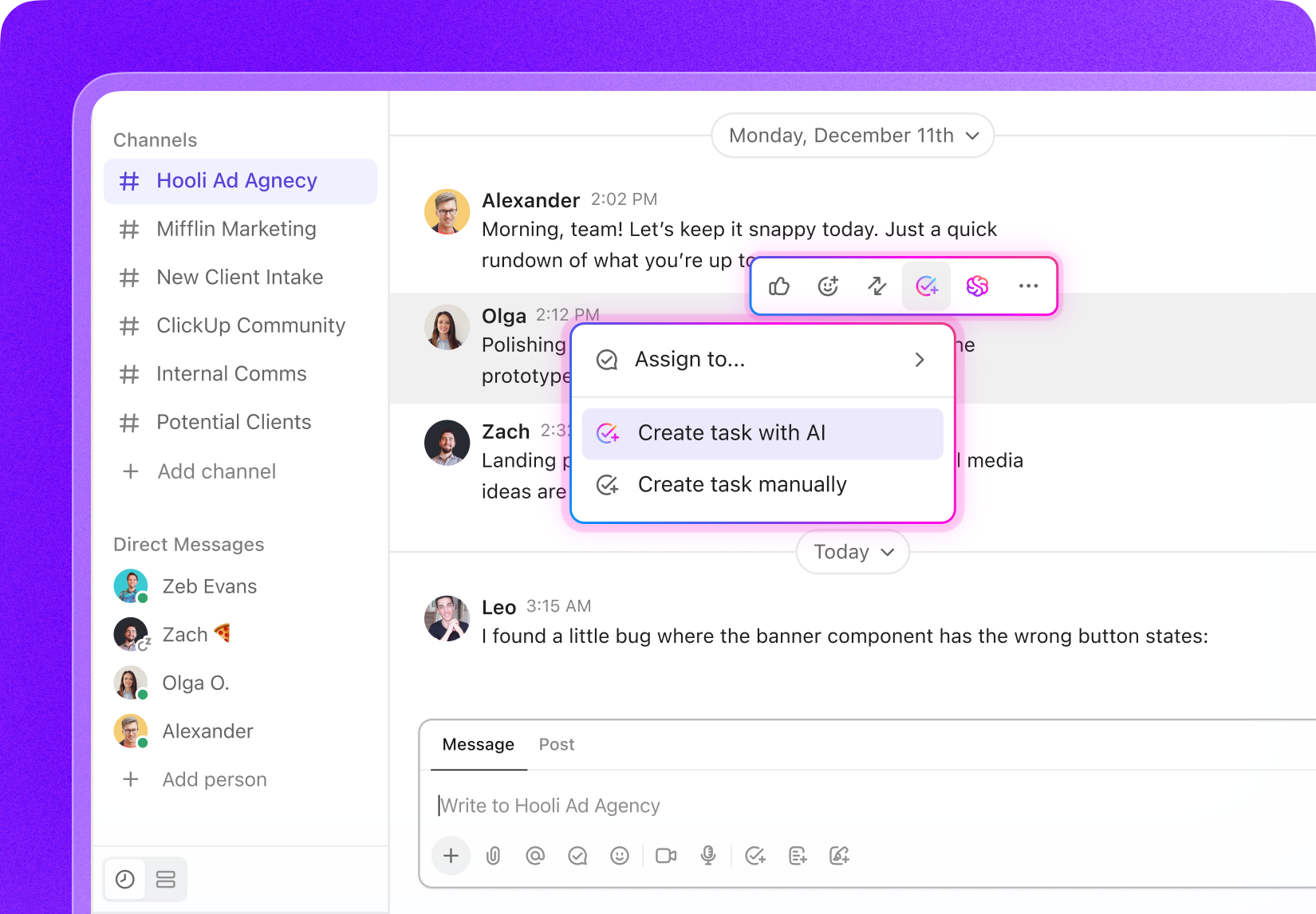
Key Stakeholders
Who Benefits Most from ClickUp in Biotech
Designed for professionals who demand clarity and control over complex biotech workflows.
If You’re a Research Scientist
Stay organized across experiments, data analysis, and protocol updates, minimizing errors and accelerating discovery.
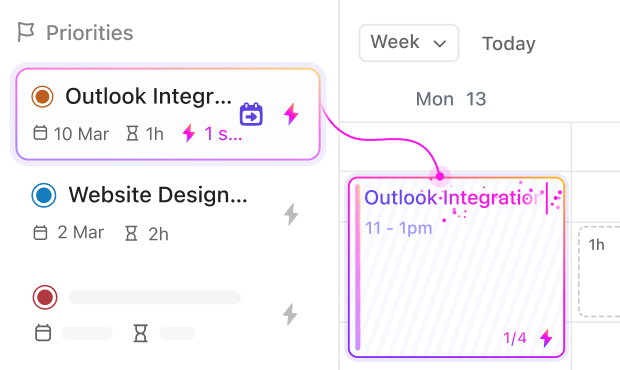
If You’re a Lab Manager
Coordinate resources, standardize procedures, and monitor compliance without juggling multiple disconnected systems.
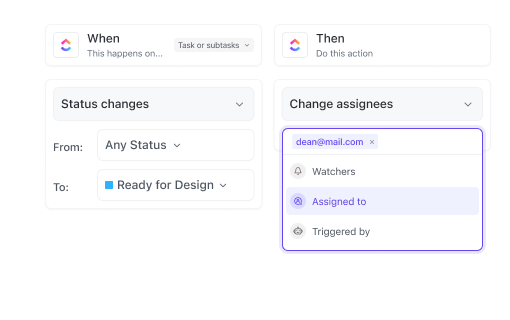
If You’re a Regulatory Affairs Specialist
Keep track of submission deadlines, documentation, and audit trails to ensure regulatory adherence with confidence.
How ClickUp Supports Biotech Success
Step-by-Step: Streamlining Your Biotech Workflow
Harness the power of ClickUp Brain and Brain Max to optimize every task and decision.
Centralize Everything
Store literature, datasets, protocols, drafts, and grant docs in one workspace — no more scattered files.
Plan Research in Phases
Break projects into proposal, literature review, experiments, analysis, and writing with task lists and Gantt timelines.
Standardize Experiments & Fieldwork
Use templates and checklists for repeatable, error-free lab or field procedures.
Collaborate Across Teams
Assign tasks to co-authors, lab members, or collaborators. Shared boards and dashboards keep everyone aligned.
Turn Meetings Into Actionable Tasks
Convert supervisor or lab meetings into tasks with owners, checklists, and deadlines.
Stay on Top of Deadlines & Funding
Track grants, conferences, and submissions with automated reminders and calendars.
Ready to Organize Your Entire Research Workflow?
Reduce chaos, prevent duplication, and focus on what matters most.