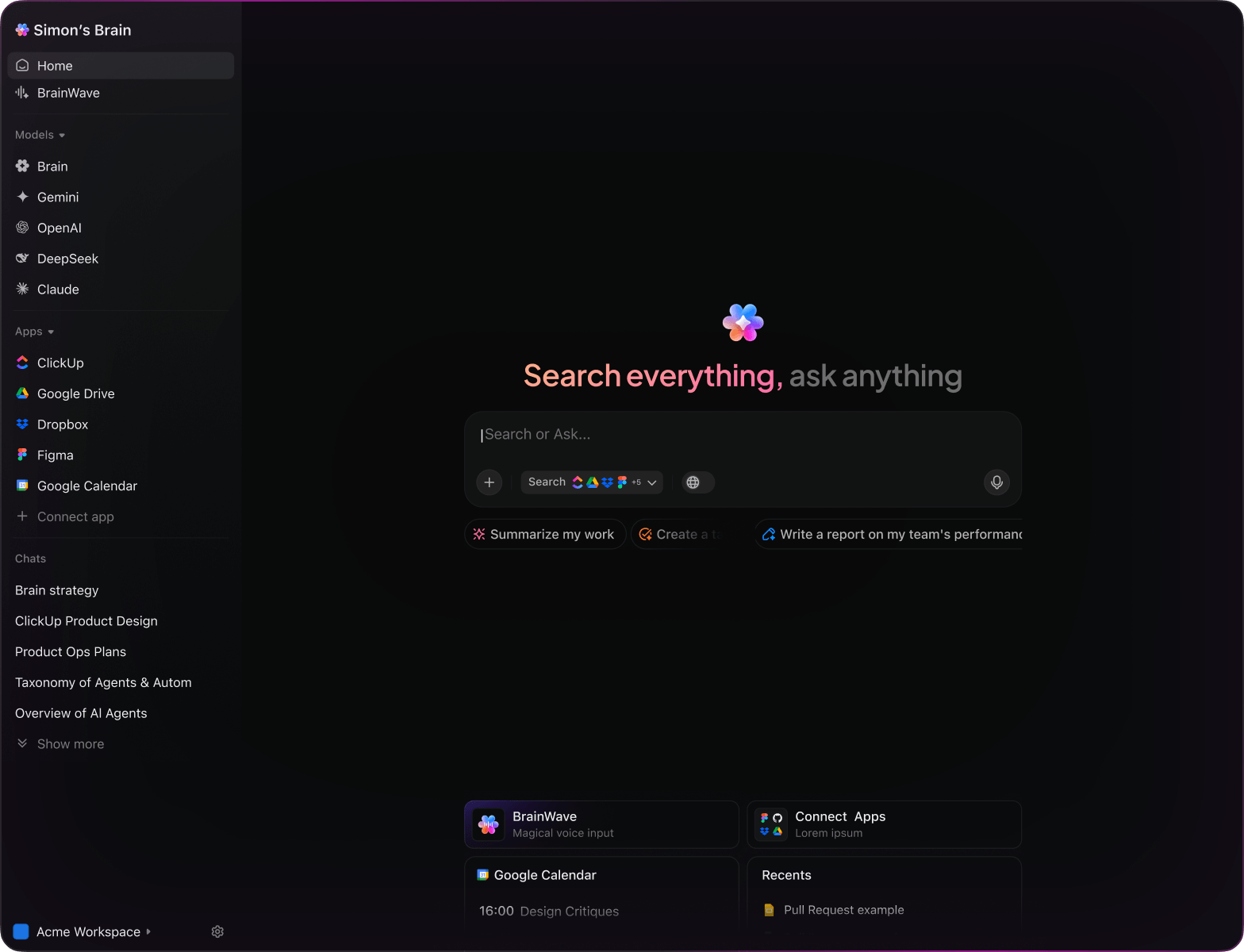
AI Tools for Regulatory Affairs
Top AI Prompts for Regulatory Affairs Teams
Simplify compliance tasks, enhance document accuracy, and accelerate regulatory submissions with ClickUp AI.

Trusted by the world’s leading businesses
AI in Regulatory Affairs
AI Prompts Revolutionizing Regulatory Affairs Workflows
Navigating the complex world of regulatory compliance demands precision and agility.
From dossier preparation to submission tracking and post-market surveillance, regulatory teams juggle numerous documents, deadlines, and evolving standards. AI prompts are now a vital asset in this demanding environment.
Regulatory professionals leverage AI to:
- Quickly extract and highlight pertinent regulatory updates
- Generate draft submissions and compliance reports with ease
- Interpret dense guidelines and summarize key points
- Transform scattered notes into clear action plans and task lists
Integrated within familiar tools—such as documents, collaboration boards, and project trackers—AI in ClickUp Brain acts as a powerful partner, converting complex regulatory data into manageable, actionable workflows.
ClickUp Brain vs Conventional Solutions
Why ClickUp Brain Stands Out
ClickUp Brain integrates seamlessly, understands your context, and empowers you to focus on action rather than explanation.
Conventional AI Platforms
- Constantly toggling between apps to collect information
- Repeating your objectives with every query
- Receiving generic, irrelevant outputs
- Hunting through multiple systems for a single document
- Interacting with AI that lacks initiative
- Manually switching among different AI engines
- Merely a browser add-on without deep integration
ClickUp Brain
- Deeply connected to your projects, documents, and team communications
- Retains your past interactions and priorities
- Delivers detailed, context-driven guidance
- Provides a unified search across your entire workspace
- Supports voice commands through Talk to Text
- Automatically selects the optimal AI model: GPT, Claude, Gemini
- Dedicated desktop apps for Mac & Windows designed for performance
Prompts for Regulatory Affairs
15 Essential AI Prompts for Regulatory Affairs Teams
Simplify regulatory workflows—compliance, documentation, and risk management made straightforward.

Identify 5 emerging regulatory trends impacting pharmaceutical approvals in the EU, based on the ‘Q2 Regulatory Update’ document.
ClickUp Brain Behaviour: Analyzes linked documents to highlight key regulatory changes and forecast trends relevant to pharma compliance.

What are the current labeling requirements for medical devices under FDA guidelines?
ClickUp Brain Behavior: Consolidates information from internal regulatory manuals; Brain Max can supplement with latest public FDA updates if accessible.

Draft a compliance checklist for clinical trial submissions referencing the ‘ICH E6(R3) Guidelines’ and prior audit reports.
ClickUp Brain Behavior: Extracts critical points from linked documents to generate a structured and actionable checklist for trial documentation.

Summarize differences in adverse event reporting between EMA and FDA using the ‘Pharmacovigilance Standards’ doc.
ClickUp Brain Behavior: Pulls tabular and textual data from internal sources to provide a clear comparative overview.

List key documentation standards for regulatory submissions in biotechnology, referencing R&D protocols and regulatory templates.
ClickUp Brain Behavior: Reviews internal documents to compile a list of essential standards and formatting guidelines.

From the ‘Device Risk Assessment’ doc, create a structured risk mitigation plan template.
ClickUp Brain Behavior: Identifies risk factors and translates them into a formatted plan within a task or document.

Summarize 3 recent changes in data privacy regulations affecting clinical data management from 2023 compliance reports.
ClickUp Brain Behavior: Extracts recurring themes and updates from linked regulatory and internal compliance documents.

From the ‘Regulatory Affairs Team Survey Q1’ doc, summarize key challenges faced in submission timelines.
ClickUp Brain Behavior: Analyzes survey data to identify common bottlenecks and areas for process improvement.

Write clear and concise regulatory communication templates for FDA correspondence using the tone guide in ‘ComplianceTone.pdf’.
ClickUp Brain Behavior: Uses style references to suggest professional and approachable language variations for official letters.

Summarize upcoming changes in EU MDR 2027 and their implications for device classification.
ClickUp Brain Behavior: Reviews linked regulatory updates to outline critical compliance shifts and design considerations.

Generate a checklist for labeling compliance specific to Canadian Health Regulations, referencing internal policy docs.
ClickUp Brain Behavior: Extracts key labeling requirements and compiles them into a practical compliance checklist.

Create a post-market surveillance plan template using US FDA guidance documents and internal case studies.
ClickUp Brain Behavior: Identifies necessary monitoring activities and structures them into an actionable plan format.

Compare regulatory submission timelines and requirements across Japan, South Korea, and Australia using competitive intelligence reports.
ClickUp Brain Behavior: Summarizes and formats comparative data into an easy-to-read table or brief.

What are the latest trends in digital health regulation since 2022?
ClickUp Brain Behavior: Synthesizes insights from recent regulatory updates, whitepapers, and internal analyses.

Summarize key compliance gaps identified in recent audits across APAC markets (documentation, process adherence, reporting).
ClickUp Brain Behavior: Extracts and prioritizes audit findings from reports, feedback notes, and corrective action plans.
Accelerate Regulatory Workflows with ClickUp Brain
Cut down on revisions, unify your compliance team, and produce superior regulatory documents using AI-driven processes.


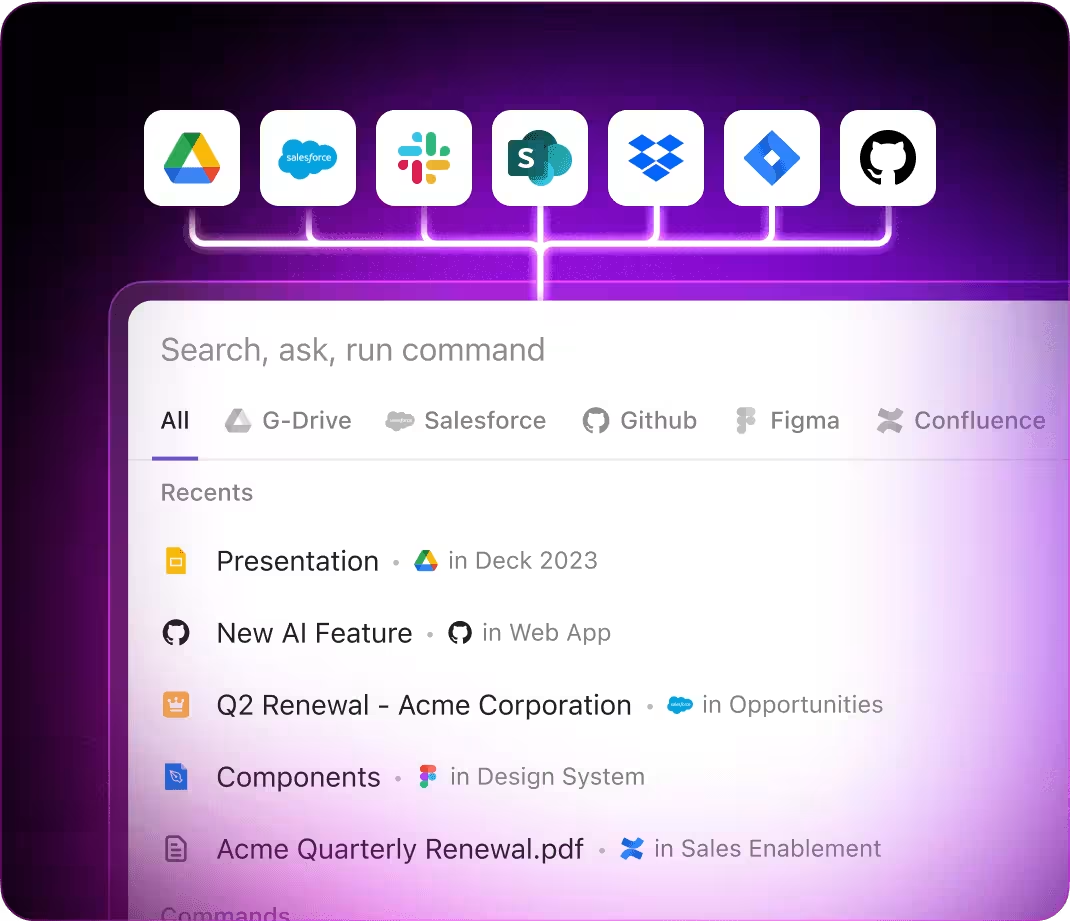
AI Prompts Tailored for Regulatory Affairs with ClickUp Brain
Discover How ChatGPT, Gemini, Perplexity, and ClickUp Brain Support Regulatory Teams
ChatGPT Regulatory Prompts
- Outline a 5-point compliance summary from recent regulatory submissions focusing on safety standards.
- Draft a regulatory update announcement highlighting new FDA guidelines and their implications.
- Generate 3 alternative risk mitigation strategies for a pharmaceutical product launch.
- Write a detailed workflow for preparing a clinical trial application ensuring all regulatory checkpoints are met.
- Compare last 3 audit reports and summarize key compliance gaps for corrective action planning.
Gemini Regulatory Prompts
- Develop 3 alternative labeling compliance checklists based on recent regulatory feedback.
- List innovative approaches to streamline document control processes with a focus on audit readiness.
- Create a mood board description for a regulatory training program emphasizing clarity, engagement, and retention.
- Suggest ergonomic layouts for regulatory submission dashboards and rank them by usability and data visibility.
- Produce a comparison table of regulatory frameworks across three regions focusing on submission timelines and requirements.
Perplexity Regulatory Prompts
- List 5 emerging regulatory trends in medical device approvals and rank them by impact.
- Provide a comparison of pharmacovigilance software options highlighting features, compliance, and cost.
- Summarize global regulatory harmonization efforts and their effects on product registrations.
- Generate a list of 5 best practices for managing regulatory correspondence and prioritize by efficiency.
- Compare previous regulatory inspection outcomes and summarize top 3 improvement areas for future audits.
ClickUp Brain Regulatory Prompts
- Transform this regulatory feedback log into prioritized compliance tasks with assigned owners.
- Summarize regulatory meeting minutes and create follow-up action items with deadlines.
- Analyze annotated submission documents and generate a checklist of outstanding regulatory requirements.
- Compile a task list from cross-department discussions on labeling updates, including priority and status.
- Summarize audit transcripts and produce actionable corrective and preventive action tasks within ClickUp.
How ClickUp Supports You
Transform Initial Thoughts into Final Plans
- Quickly convert scattered notes into polished regulatory documents.
- Generate innovative strategies leveraging historical compliance data.
- Build standardized templates to accelerate regulatory submissions.
Brain Max Boost: Effortlessly access previous filings, expert reviews, and regulatory guidelines to guide your next submission.
Why ClickUp Works for You
Accelerate Regulatory Submissions
- Break down regulatory meetings into precise, actionable items.
- Transform compliance feedback into organized, assignable tasks.
- Automatically create audit trails and submission summaries without extra effort.
Brain Max Boost: Quickly access historical filing data, regulation cross-references, or document versions across your projects.
AI Advantages
How AI Prompts Elevate Every Phase of Regulatory Affairs
AI prompts accelerate compliance workflows and empower smarter regulatory strategies.
Quickly Develop Regulatory Strategies
Regulatory teams explore diverse approaches rapidly, make informed compliance choices, and prevent bottlenecks.
Enhance Compliance Accuracy
Improve decision-making, reduce regulatory risks, and ensure submissions meet agency expectations.
Identify Issues Early to Avoid Delays
Detect potential compliance gaps sooner, minimize costly revisions, and speed approval timelines.
Align Cross-Functional Teams Efficiently
Facilitates clear communication, prevents misunderstandings, and accelerates consensus among regulatory, legal, and product teams.
Drive Innovative Regulatory Solutions
Encourages creative problem-solving, adapts to evolving regulations, and maintains competitive advantage.
Integrated AI Support Within ClickUp
Transforms AI-generated insights into actionable tasks that advance regulatory projects seamlessly.
Boost Your Regulatory Workflow
Cut down mistakes, simplify approvals, and generate precise documents with AI-powered support.